Depending on the size of the pores engineered into the membrane, crossflow filters are effective in the classes of separation known as reverse osmosis, nanofiltration, ultrafiltration and the more recent microfiltration.
Reverse Osmosis Plant (RO Plant)
Reverse Osmosis Plant (RO Plant)
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water.
Reverse osmosis, invented in 1959, is the newest major method of water purification and one of the types of crossflow membrane filtration. It is a process which removes both dissolved organics and salts using a mechanism different from ion exchange or activated carbon. The pressurized feedwater flows across a membrane, with a portion of the feed permeating the membrane. The balance of the feed sweeps parallel to the surface of the membrane to exit the system without being filtered. The filtered stream is the “permeate” because it has permeated the membrane. The second stream is the “concentrate” because it carries off the concentrated contaminants rejected by the membrane. Because the feed and concentrate flow parallel to the membrane instead of perpendicular to it, the process is called “crossflow filtration”.
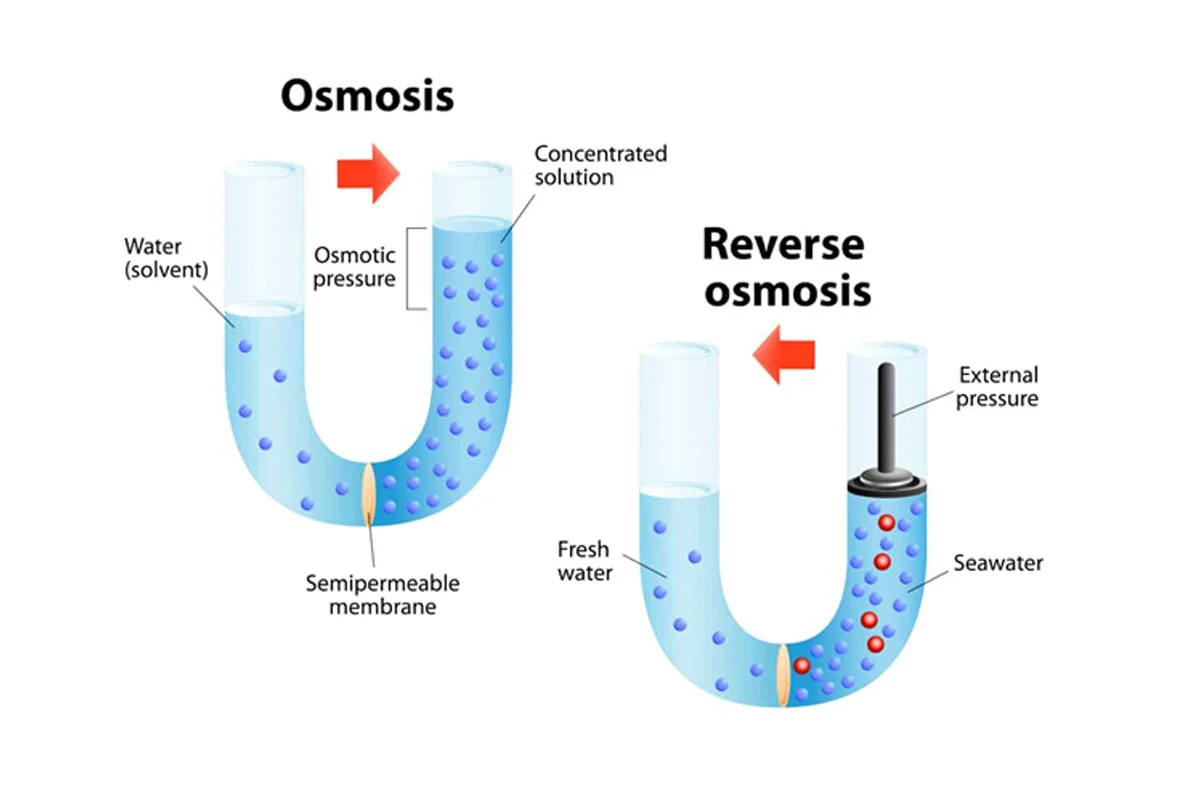
Crossflow membrane filtration allows continuous removal of contaminants which in “normal flow” filtration would “blind” (cover up) or plug the membrane pores very rapidly. Thus the crossflow mode of operation is essential to these processes.
Reverse osmosis (RO) was the first crossflow membrane separation process to be widely commercialized. RO removes most organic compounds and up to 99% of all ions. A selection of RO membranes is available to address varying water conditions and requirement.
RO can meet most water standards with a single-pass system and the highest standards with a double-pass system. This process achieves rejections of 99.9+% of viruses, bacteria and pyrogens. Pressure in the range of 50 to 1000 psig (3.4 to 69 bar) is the driving force of the RO purification process. It is much more energy-efficient compared to phase change processes (distillation) and more efficient than the strong chemicals required for ion exchange regeneration.
Reverse osmosis plants require a variety of pre-treatment techniques including softening, dichlorination, and anti-scalent treatment. Following pre-treatment, high levels of pressure send water through a semi-permeable membrane, which retains all contaminants but let’s pure water pass through.
Advantages of Reverse Osmosis
- Some of the advantages of the reverse osmosis process are as follows
- It is the best method for water softening.
- The semipermeable membrane will block all ion particles.
- Maintenance of the system is very simple.
- It gives us clean and pure water by blocking all contaminants.
- The available RO systems are very compact, and it requires little space.
- The useful life of the full system, including the membrane, is over two years.
- This system does not require any use of chemicals to purify water.
- The energy requirement for the RO system is very low.
- RO systems are totally automated and are designed to start and stop on their own.
Applications of Reverse Osmosis
Reverse osmosis is widely used in the residential and commercial water filtration system. Other than that, it has plenty of applications in various industries. Some of them are mentioned below
- Reverse osmosis is a type of a process which is used to remove dissolved chemical particles from water.
- Reverse osmosis is a type of a process which is used to remove dissolved biological entities from water.
- It is highly used in desalinating seawater.
- It has crucial applications in the medical field.
- It is used to purify water to prevent any diseases.
- It has a wide application in water treatment and water purification.
- It is used in food industries, and it is applied for the concentration of juices, milk, and other beverages.
- It is used to provide clean water for the community water supply.

